“Selected Articles in English”
Selected articles originally published in Korean are now
translated into English,
making them accessible to the readers worldwide.
Does everything happen for a reason, as suggested by Aristotle? This may be the case if we accept that such a cause or purpose is not necessarily human-centered. However, even if that were the case, there is no guarantee that it would always be humanly recognizable. Nonetheless, humanity has long pursued an understanding of any existence and phenomena that are accessible. These efforts have involved significant modifications of concepts and ideas, and sometimes drastic revisions of the worldview, transcending pre-existing ones so as to reach the realm of new facts. Among all such endeavors, the discovery of quantum theory in the early 20th century is one of the most striking developments, and reflects truly revolutionary breakthroughs in human concepts and reasoning. It was a manifestation of the greatness of human intellectual power; it literally provided unprecedented answers to all questions about the material world.
No one knows for sure whether quantum theory will withstand the test of time and will indeed remain the ultimate law explaining the world. However, it is true that currently known quantum mechanical laws are effective and accurate in describing all atomistic and molecular states and processes, as have been proven by the accumulation of facts confirmed over the past hundred years. In this sense, everything that exists on Earth is based on quantum phenomena. However, this general fact is not what is typically referred to as quantum phenomena. Rather, quantum phenomena or effects conventionally mean the apparent manifestation of quantum principles that cannot be explained by classical mechanics at all. Prime examples of these include the superposition and nonlocality of quantum states.
Without photosynthesis, there is no ecological system on Earth and thus no humanity. Thus, photosynthesis is the foundation of all life processes that we know of. Research on photosynthesis dates back to the late 16th century. However, the basic mechanism of photosynthesis – namely, the fact that light enables the conversion of carbon dioxide and water into oxygen molecules and key organic molecules, which serve as fuels for plants – was established only in the late 18th century [2].
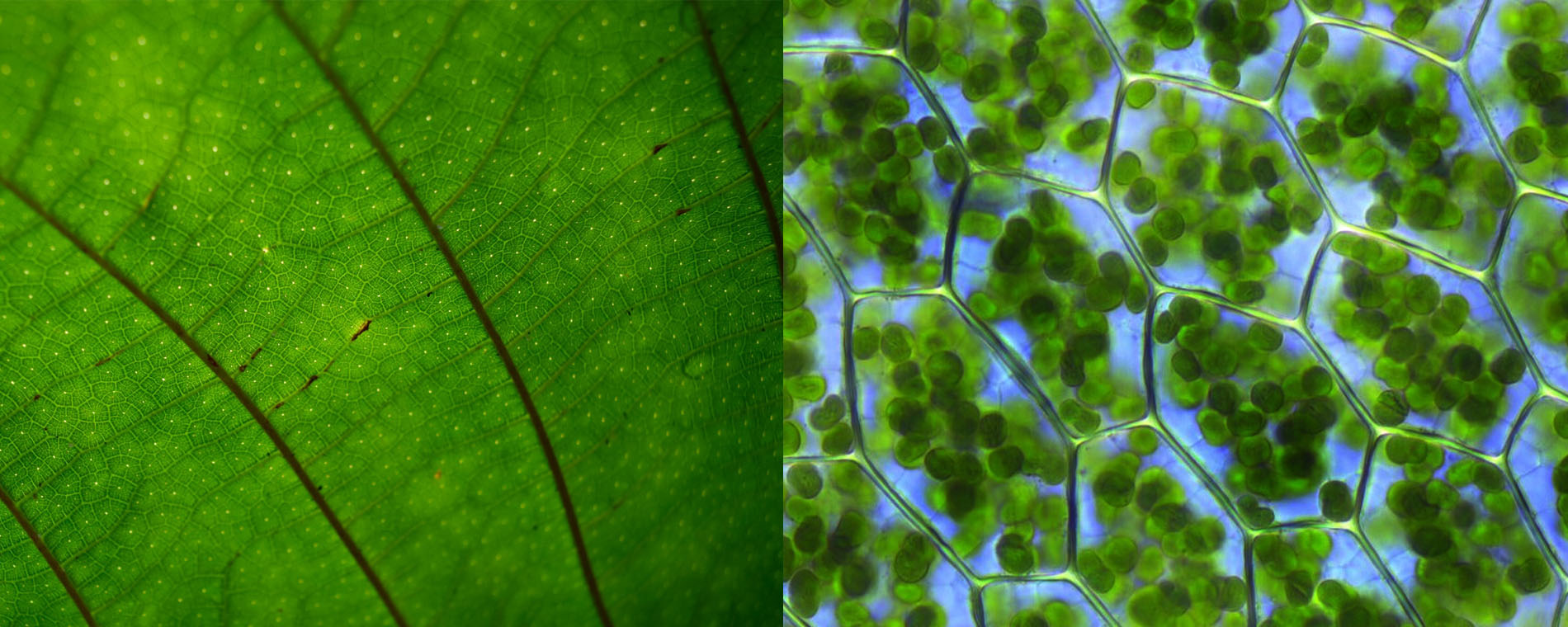
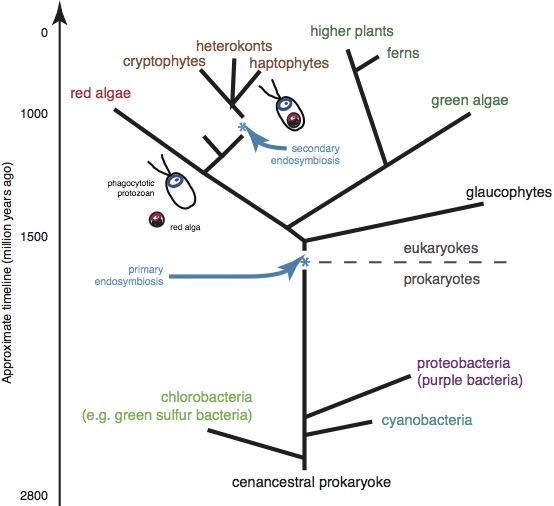
Photosynthesis is not “photo-synthesis” in a simple sense. If it were, issues of energy and global warming would have been solved a long time ago. Rather, it is a remarkable collection of physical and chemical processes occurring at the cellular scale that are initiated by the absorption of light. Prior to the chemical reaction stage of photosynthesis, energy attained from light has to be transported to specific domains, through highly sophisticated processes that occur on nanosecond time scales. These processes are mysteriously efficient. It turns out that such efficiency can be understood only when the concepts of quantum mechanical superposition and nonlocality are recognized. It is also remarkable that the appropriate arrangement of effective molecules and proteins, which makes such processes possible, was discovered and maintained by bacteria, algae, and plants through billions of years of evolutionary processes (see Fig. 1). In contrast, even the most advanced methods of chemistry and physics available today are still too rudimentary to mimic or recreate such highly evolved natural processes in any synthetic manner.
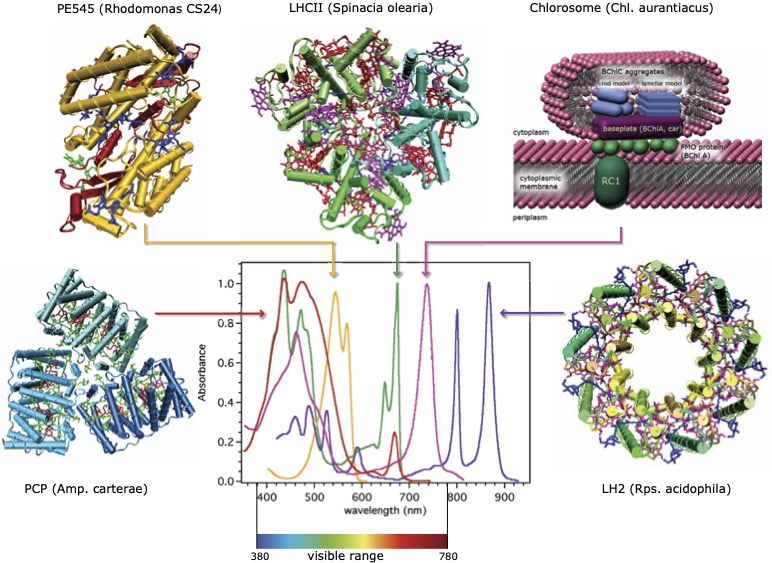
All photosynthetic organisms have light-harvesting complexesLHCs that absorb, retain, and transport the energy from light. These LHCs generally consist of pigment molecules embedded in complex three-dimensional protein structures. Figure 2 shows some important examples of LHCs.
Various photosynthetic organisms have evolved to adapt to different environments, and thus have developed distinct LHCs optimized to efficiently absorb and process light energy under their living conditions. Above all, the wavelengths of light absorbed by different LHCs have become highly optimized. Considering that most LHCs have chlorophylls or their variants as major pigment molecules, the apparent diversity is striking. This is because those pigment molecules all absorb at similar wavelengths in isolated or simple solution environments. It has long been speculated that the primary mechanism for the tuning of wavelengths is the modulation of the local electronic environments of pigment molecules by surrounding protein residues. However, only recently have calculations been able to describe such tuning effects on the basis of fully quantum mechanical principles. As yet, current level of quantum chemistry calculations is not sufficiently accurate to independently account for experimental observations. Moreover, with the present status of materials synthesis and fabrication, it remains far-fetched to recreate such effects of proteins synthetically. Thus, further advances are needed and progress is actively being made.
The light energy absorbed by molecules typically gets lost irreversibly, either through the emission of light with lower energy or the generation of heat. Thus, for the energy to be utilized, it must be transported quickly and safely to appropriate environments for creating fuels that organisms can easily store and use. Such transport processes are carried out by pigment molecules in LHCs. With the exception of the chlorosome, most LHCs consist of $\sim 10 – 100 $ pigment molecules arranged at appropriate distances and angles from each other. Typically, nearest-neighbor distances of pigment molecules are about 1 nanometer, and the sizes of LHCs are in the range of 5-10 nanometers. These LHCs can in turn form larger supercomplexes, which serve as efficient overall pathways for the transport of energy.
Then, what is the detailed mechanism of energy transport? To understand this, it is necessary to first discuss a mechanism called resonance energy transfer. Individual molecules can absorb light and then emit lower-energy light through fluorescence or phosphorescence. All these processes involve transitions between different quantum mechanical states of electrons in molecules.1 [4]
Absorption of light occurs when electrons in the ground state make transitions to excited states, whereas fluorescence or phosphorescence involves transition of excited states to lower (typically ground) electronic states. If an excited molecule is near a different molecule in the ground state, a new route for energy transfer between the two can open up. Through quantum mechanical resonance of electronic states, an excited state molecule can return to the ground state while exciting the nearby molecule that was originally in the ground state. This kind of energy transfer mechanism was established in 1948 by the German theoretician Förster [5], and has continued to be developed by others further since then.
The possibility that Förster’s theory could explain energy transfer in LHCs was first suggested in the 1950s, and various experimental reports have reinforced this view at least qualitatively. As a prime example, it was demonstrated that the energy transfer between pigment molecules separated by around 1- 2 nanometers, which are typical distances between nearest-neighbor pigment molecules in LHCs, can occur within about 1 picosecond$10^{-12}$ second. However, concrete experimental investigations and theoretical studies became possible only during the past three decades. This was enabled by the determination of LHC structures through modern X-ray crystallography and by advances in laser spectroscopy that allowed measurements down to the femtosecond$10^{-15}$ second time scale. When these experimental data were analyzed carefully with modern theoretical methods, it became clear that actual energy transfer generally happens much faster than what Förster’s theory predicted. A representative example can be found in an LHC called light-harvesting 2LH2, which plays a central role in the photosynthesis by purple bacteria.
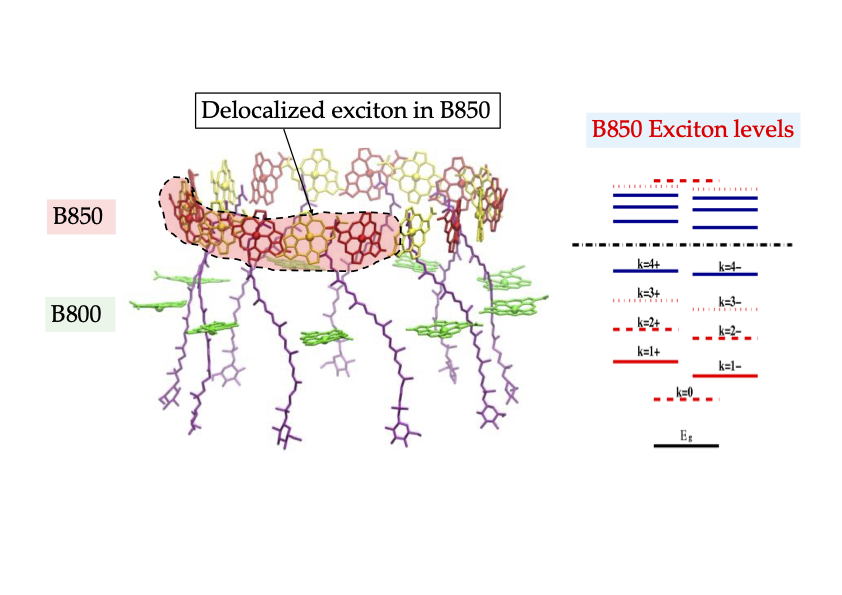
Purple bacteria are ancient photosynthetic organisms that are believed to have evolved more than 2.5 billion years ago2 [6] but are still thriving. In these organisms, LH2s are responsible for the collection and transport of light energy during the early stage of photosynthesis. LH2 consists of B800 and B850 units. The former and the latter absorb light at wavelengths around 800 and 850 nanometers, respectively. Both are composed of circularly arranged bacteriochlorophyllsBChls as pigment molecules (see Fig. 3). In particular, BChls in the B850 unit are closely packed, with nearest neighbors about 1 nanometer apart. The nature of electronic excited states formed in this B850 unit is truly quantum mechanical. These states, known as excitons, are quantum mechanical superpositions of those of individual molecules. Excitons still count as one excitation but have properties of more than one BChl. They are indeed prime examples of delocalized quantum mechanical superposition states. [1]
The quantum nature of excitons is critical for efficient energy transfer in LHCs (see Fig. 3). For example, the superposition makes it possible to extend the domain of excitation beyond a single pigment molecule in intrinsically quantum mechanical ways. Such quantum delocalization enables enhancement of energy transfer rates from the B800 unit to the B850 unit within an LH2 complex by about a factor of seven [7, 8], when compared with the prediction of Förster’s theory. The same effects have also been shown to enhance the energy transfer rate between different LH2 complexes by about a factor of four [9]. These calculations are consistent with experimental data. What is also remarkable is that the superposition states are utilized positively for flexible and robust energy transfer in complex, disordered, and fluctuating biological environments. This is because superposed states are easily deformable and can adapt to different environments in ways surpassing classical states. Such enhanced energy transfer rates involving LH2 complexes in turn extend the overall distances of the energy transport in the aggregates of LH2 complexes, which are estimated to be about 100 nanometers. These distances are ten times larger than those observed in even the most efficient organic photovoltaic devices currently available, and explain why natural LHC complexes form superior energy transport pathways.
Research on the physics and chemistry of photosynthesis is more than a hundred years old, but the progress during the past thirty years has been groundbreaking thanks to advances in spectroscopy, nanoscience, and computational sciences. In particular, these advances made it possible to elucidate mechanistic and quantitative details of the energy transfer down to the molecular level. The broader interest in Quantum Biology has also made great contributions to these advances. Indeed, the extensive interest in Quantum Biology that started around 2007 was directly motivated by pioneering femtosecond nonlinear laser spectroscopy experiments on LHCs. [10, 11] However, it is also important to note that many of the fascinating quantum phenomena proposed as potential mechanisms to explain new spectroscopic experiments on LHCs, although brilliant theoretical proposals, turned out not to be possible or significant through more detailed calculations and experiments. This assessment is not yet definitive and may reflect my subjective viewpoint, but is something the majority of experts now agree on. Nonetheless, what is really important and often overlooked is the fact that quantum phenomena associated with superposition and delocalization indeed likely play fundamental roles in photosynthesis, which are now beyond question thanks to confirmations through accurate calculations and experiments. The core reason for the difficulty of directly observing these quantum phenomena in natural LHCs is that they are hidden under disorder and fluctuations, unlike those observed in engineered synthetic systems.
Is it really surprising to find that quantum phenomena make important contributions to photosynthesis? The answer to this question can be both yes and no. On one hand, the confirmation of quantum phenomena is natural and unsurprising. On the other hand, it is truly fascinating. The reason it is not surprising is that even biological entities are governed by quantum mechanical laws at molecular length and time scales. If not, it would mean that quantum mechanics cannot capture all aspects of materials and their processes, contrary to what has been confirmed during the past 100 years. The reason it is fascinating is that it demonstrates that even bacteria utilize subtle quantum effects in a balanced and practical manner for their survival. The initial stage of photosynthesis is what natural organisms figured out through long evolutionary processes, and it reveals the secret of how intrinsically quantum mechanical light can be harnessed positively even in complex environments. This also suggests that deeper research into how such an ability can be realized synthetically may lead to new advances in light-energy technologies.
References
- S. J. Jang and B. Mennucci, Delocalized excitons in natural light harvesting
complexes, Rev. Mod. Phys. 90, 035003 (2018). - R. E. Blankenship, Molecular Mechanisms of Photosynthesis, 2nd Ed. (Wiley
Blackwell, Oxford, 2014). - G. D. Scholes, Quantum-coherent electronic energy transfer: Did nature think of
it first?, J. Phys. Chem. Lett. 1, 2 (2010). - In general, light with wavelength in the near infrared region or shorter wavelength is required to excited electronic states of molecules.
- T. Förster, Intermolecular energy migration and fluorescence, Ann. Phys. (Berlin) 437, 55 (1948).
- There is no satisfactory agreement on this estimate yet.
- S. Jang, M. D. Newton, and R. J. Silbey, Multichromophoric Förster resonance energy transfer, Phys. Rev. Lett. 92, 218301 (2004).
- S. Jang, M. D. Newton, and R. J. Silbey, Multichromophoric Förster resonance energy transfer from B800 to B850 in the light harvesting complex 2: Evidence for subtle energetic optimization by purple bacteria, J. Phys.
Chem. B 111, 6807 (2007). - S. J. Jang, Robust and fragile quantum effects in the transfer kinetics of delocalized excitons between LH2 complexes, J. Phys. Chem. Lett. 9, 6576 (2018).
- G. S. Engel, T. R. Calhoun, E. L. Read, T.-K. Ahn, T. Mancal, Y.-C. Cheng, R. E. Blankenship, and G. R. Fleming, Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems, Nature 446, 782
(2007). - E. Collini, C. Y. Wong, K. E. Wilk, P. M. G. Curmi, P. Brumer, and G. D. Scholes, Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature, Nature 463, 644 (2010).